Climara Pro®—proven efficacy for moderate to severe vasomotor symptoms
Meet Jill
- Postmenopausal at the age of 51 years
- Looking for an option to manage her moderate to severe vasomotor symptoms
- Has an intact uterus
Hypothetical patient for illustrative purposes only.

Jill is postmenopausal and recently has been experiencing frequent and unpredictable moderate to severe vasomotor symptoms.
During a recent visit with her healthcare provider, she complained of worsening symptoms and discussed her need for a treatment that will help manage her condition.
Review clinical data on a treatment option below.

Meet Jill
- Postmenopausal at the age of 51 years
- Looking for an option to manage her moderate to severe vasomotor symptoms
- Has an intact uterus
Hypothetical patient for illustrative purposes only.
Jill is postmenopausal and recently has been experiencing frequent and unpredictable moderate to severe vasomotor symptoms.
During a recent visit with her healthcare provider, she complained of worsening symptoms and discussed her need for a treatment that will help manage her condition.
Review clinical data on a treatment option below.
Hypothetical patient for illustrative purposes only.
Demonstrated efficacy in a clinical trial
The efficacy of 0.045 mg of estradiol/0.03 mg levonorgestrel administered weekly versus placebo in the relief of moderate to severe vasomotor symptoms in postmenopausal women was studied in a 12-week clinical trial (N=183, average age 52 years).
Climara Pro® significantly reduced the number of daily moderate to severe hot flushes at week 12 compared with placebo

*P<0.001 versus placebo.d
At baseline
The mean number of moderate to severe hot flushes was 10.13 per day for the treatment group (nc=92) and 10.8 for the placebo group (nc=88)
At week 4
73% reduction in the mean number of moderate to severe hot flushes from baseline for the treatment group (nc=88) and 43% for the placebo group (nc=82) (P<0.001)d
At week 8
The mean number of moderate to severe hot flushes was 1.22 per day for the treatment group (nc=80) and 5.35 per day for the placebo group (nc=73)
At week 12
90% reduction in the mean number of moderate to severe hot flushes from baseline for the treatment group (nc=73) and 48% for the placebo group (nc=69) (P<0.001)d
E2=estradiol; LNG=levonorgestrel.
aITT=intent-to-treat population.
bClimara Pro® and the 0.045 mg estradiol/0.03 mg levonorgestrel dosage strength are bioequivalent in terms of estradiol delivery.
cn=Number of subjects in a treatment group in a cycle; number of subjects varied from cycle to cycle due to missing data.
dP value for comparison with placebo, adjusted by the method of Bonferroni; P<0.025.
Climara Pro® significantly reduced the severity of moderate to severe hot flushes at week 12 versus placebo
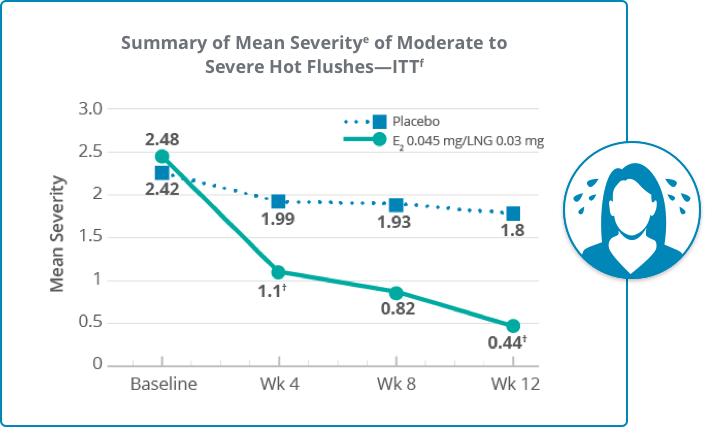
*P<0.001 versus placebo.d
At baseline
The mean severity of moderate to severe hot flushes was 2.48 per day for the treatment group (nh=92) and 2.42 for the placebo group (nh=89)
At week 4
56% reduction in the mean severity of moderate to severe hot flushes from baseline for the treatment group (nh=83) and 18% for the placebo group (nh=76) (P<0.001)i
At week 8
The mean severity of moderate to severe hot flushes was 0.82 for the treatment group (nh=72) and 1.93 for the placebo group (nh=68)
At week 12
82% reduction in the mean severity of moderate to severe hot flushes from baseline for the treatment group (nh=55) and 26% for the placebo group (nh=57) (P<0.001)i
eSeverity scores are: 1=Mild, 2=Moderate, 3=Severe. Mean severity of hot flushes by day is ([2X number of moderate hot flushes] + [3X number of severe hot flushes]) / total number of moderate to severe hot flushes on that day. If no moderate to severe hot flush was indicated, the mean severity was 0.
fITT=intent-to-treat population.
gClimara Pro® and the 0.045 mg estradiol/0.03 mg levonorgestrel dosage strength are bioequivalent in terms of estradiol delivery.
hn=Number of subjects in a treatment group in a cycle; number of subjects varied from cycle to cycle due to missing data.
iP value for comparison with placebo, adjusted by the method of Bonferroni; P<0.025.
Adverse reactions
In a prospective, randomized, placebo-controlled, double-blind study, the most common adverse reactions ≥5% were: application site reaction, vaginal bleeding, breast pain, upper respiratory infection, back pain, depression, pain, and headache.



